BellaSeno, a German medical startup, has obtained ISO 13485 certification for its 3D printed breast implants, branded as Senealla. The company has also announced that is has raised a further €1 million from existing investors, totaling €4.2 million in funding.
According to Simon Champ, CEO of BellaSeno, the funding and certification will enable clinical trials of Senella. Champ added, “We are offering the entire process under ISO 13485 certification to other medtech companies worldwide, from concept and in-house design to manufacturing of prototypes, clinical trials and series production.”

Senella technology
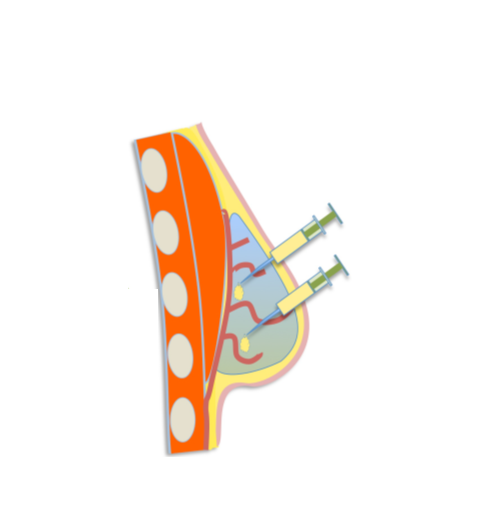
Since its founding in 2015, BellaSeno has worked to develop an alternative to silicone breast implants which permanently introduce a foreign body into the patient. Thus, Senella technology was introduced and produces porous, polycaprolactone (PCL) scaffolds that can be resorbed by the body.
Augmentation of the breast is achieved as fat tissue is harvested via liposuction and injected via lipofilling into the scaffold. This provides a stable platform for the tissue to mature over a two-year span, resulting in natural breast free of foreign materials and any complications concerning silicone implants.
BellaSeno believes that Senella can disrupt breast reconstruction and augmentation technology. The first clinical trial for the breast implant is expected to begin in the fourth quarter of 2019.
Additive manufacturing and breast reconstruction
Elsewhere, additive manufacturing has been used in breast reconstruction and augmentation. Last year, plastic surgeons at Radboud University in the Netherlands discussed the applications and limitations of using patient-specific 3D prints as intraoperative guides.
This research focuses on autologous “flap” reconstruction, where tissue from one part of the body, such as the abdomen or thigh, is used as material to rebuild another body part. This is a common practice for all types of breast correction surgeries, in cases of asymmetry and augmentation, or following tumor removal.
FDA researchers have also developed patient-specific 3D printed breast phantoms which are used as test beds for mammography devices to ensure optimized breast cancer detection and treatment.

Get all the latest 3D printing news direct to your inbox, subscribe to the Industry newsletter. Also, follow us on Twitter, and like us on Facebook.
Looking for a change of pace? Seeking new talent for your business? Search and post Jobs for opportunities and new talent across engineering, marketing, sales and more.
Featured image shows a 3D printed breast implant. Photo via BellaSeno.

Leave A Comment