Kentucky-based 3D bioprinting company Koligo Therapeutics (ASX:KOL) is due to list on the Australian Securities Exchange at the end of March through an Initial Public Offering (IPO).
Koligo is hoping to raise up to AUD$7m at 20c a share from the IPO, establishing a market value of approximately AUD$22m. Koligo will use the funds raised through the IPO to expand its manufacturing capacity and drive sales into targeted hospitals, so that medical treatments can utilise Koligo technology. Furthermore, Koligo is looking to take advantage of opportunities for international growth, and to develop its patented 3D-V technology platform and product pipeline.
Matthew Lehman, CEO of Koligo, explains, “Our listing on the ASX will accelerate Koligo’s growth and improve our ability to make a real difference in patients’ lives who suffer these debilitating conditions. The local market understands the value these companies provide. We are heartened by the success of other regenerative medicine and cell therapy companies on the ASX, such as Exopharm, Polynovo and Cynata.”
Engineering tissue and cell transplantation with 3D bioprinting
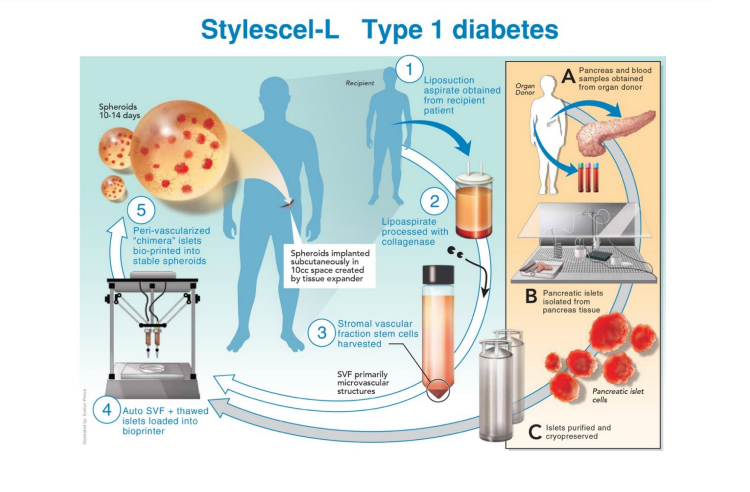
Koligo’s 3D-V bioprinting platform is intended to develop and improve its regenerative medicine products and engineer cell and tissue transplantation with 3D bioprinting technology. The 3D bioprinted products are planned for commercial release and will be used to treat serious health conditions and disorders such as pancreatitis, type 1 diabetes, liver failure and more. An example of Koligo’s 3D bioprinting processes includes Stylescel-L, where a 3D bioprinted spheroid is injected into patients to allow better oxygen and blood flow. Lehman adds:
“Koligo will transform human cell and tissue transplant with our 3D-V bio-printing. We will create 3D biodegrable bio-printed spheroids which contain a patient’s stromal vascular fraction cells and/ or micro vascular fragments and pancreas islets […] These spheroid ‘scaffolds’ allow improved function of the cell or tissue after transplant and safely biodegrade.”
Koligo is also committed to the wide distribution of pancreatic islets, which are the cells that produce insulin in the pancreas. The company distributes the islets as part of its Kyslecel product, to help treat pancreatitis, a condition resulting in the inflammation of the pancreas. Koligo partners with hospitals to remove and transfer a patient’s pancreas to its FDA-registered labs. The pancreatic islets are then preserved into ‘Kyslecel’, and sent back to hospitals to be implanted into the patient’s liver, in order to regulate blood sugar levels and treat the condition.
The company is at revenue stage with its Kyslecel product, where it is qualified for sale in the United States. Koligo estimates Kyslecel to have an annual addressable market of AUD$300m in the U.S., with the product already having generated AUD$1.6m revenue for Koligo in the first 14 months of sale from a limited number of hospital customers. By mid-2019, Koligo aim to release Kyslecel V2.0, which will maintain an improved shelf-life, allowing extended transport times for further regions and possible international distribution. With Kyslecel V2.0, Koligo estimates the US market size to be AUD$600m per year, and the company has plans to enter the Indian and Chinese markets as well.
“The location of the Koligo lab is key as Louisville, Kentucky is also the home of the UPS Worldport hub with 318 flights daily and the capability for critical shipments of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCTPs). Based on initial success of Kyslecel™, Koligo will scale production and distribution to deliver patients access to this procedure at more hospitals across the United States.”
A step forward for 3D bioprinting
Koligo’s IPO and listing on the ASX represents the latest advancement for the commercialization of 3D bioprinting.
Chicago-based BIOLIFE4D, another biotechnology company that filed an IPO of $50 million in September 2017, successfully demonstrated the ability to 3D print human heart tissue in June 2018. The company eventually hopes to be 3D printing valves, blood vessels, miniature organs and a full human heart.
Furthermore, California-based 3D bioprinting company Organovo Holdings Inc is due to meet with the Food and Drug Administration (FDA) this year on its development of 3D bioprinted tissues for the treatment of liver diseases. In 2016 the company previously suggests that its 3D bioprinted liver tissue could make it to the FDA by 2019.
Subscribe to the Industry newsletter for the latest news in additive manufacturing. You can also keep connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit Jobs for a selection of roles in the industry.
Featured image shows Koligo’s 3D-V technology. Screengrab via Koligo.

Leave A Comment